PharmaSugar® Parenteral
Meeting the needs of the market
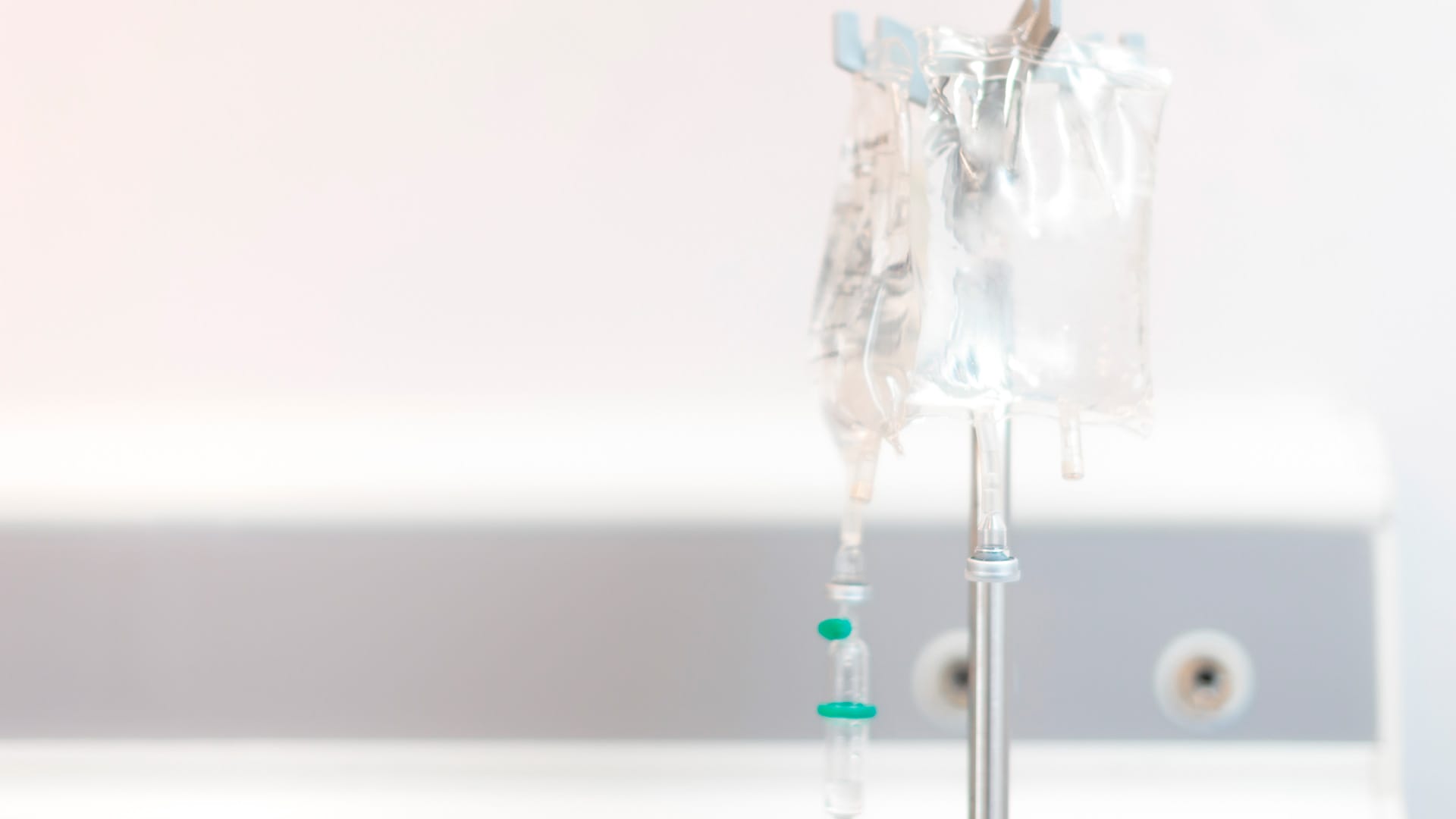
Injectable sugar for sterile preparations
PharmaSugar® is designed for the parenteral product sector. Production takes place in sugar processing plant certified to ISO 9001, FSSC 22000 and ISO 1400, and that is GM-free and registered with the national medicine authorities (France’s ANSM). PharmaSugar® batch management guarantees optimum tracing, from sourcing to delivery.
Parenteral application
PharmaSugar® sugar products cover a complete spectrum of preparations: injectable gels, diluents, injectable powder mixes and drips.
Origins and conformance
Our sugars are made in France. The Cristalco PharmaSugar® range conforms to the European Pharmacopeia and to IPEC (International Pharmaceutical Excipients Council) regulations and meets parenteral requirements.
Exemplary Traceability
Production of our sugars is managed in batches guaranteeing optimum traceability, from production to delivery. In addition, supplementary analysis for batch release (dextrins, endotoxins, etc) can be arranged to your requirements.